
Phospholipase C-γ (PLC-γ) binds to activated receptor protein-tyrosine kinases via its SH2 domains. This change in cGMP level in retinal rod cells is translated to a nerve impulse by a direct effect of cGMP on ion channels in the plasma membrane, similar to the action of cAMP in sensing smells.Īctivation of phospholipase C by protein-tyrosine kinases. Rhodopsin then activates the G protein transducin, and the α subunit of transducin stimulates the activity of cGMP phosphodiesterase, leading to a decrease in the intracellular level of cGMP. Rhodopsin is activated as a result of the absorption of light by the associated small molecule 11- cis-retinal, which then isomerizes to all- trans-retinal, inducing a conformational change in the rhodopsin protein. The photoreceptor in rod cells of the retina is a G protein-coupled receptor called rhodopsin ( Figure 13.23). The best-characterized role of cGMP is in the vertebrate eye, where it serves as the second messenger responsible for converting the visual signals received as light to nerve impulses. The action of cGMP is frequently mediated by activation of a cGMP-dependent protein kinase, although cGMP can also act to regulate other targets, including ion channels.
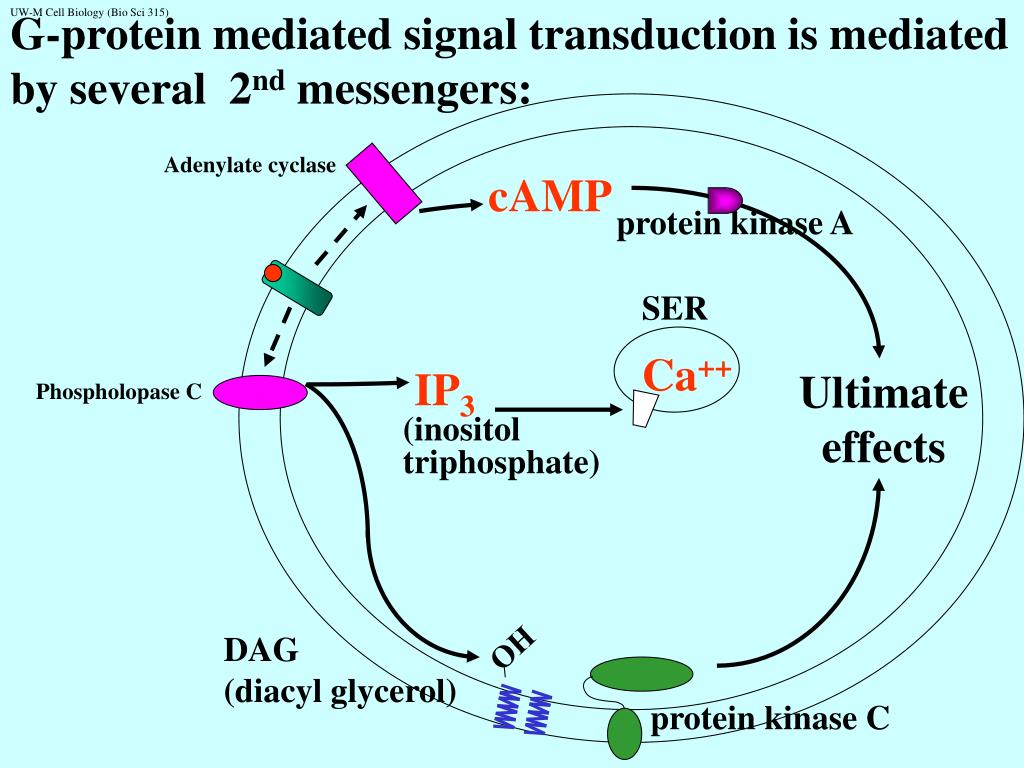
Stimulation of these guanylyl cyclases leads to elevated levels of cGMP, which then mediate biological responses, such as blood vessel dilation. As discussed earlier in this chapter, different types of guanylyl cyclases are activated by both nitric oxide and peptide ligands. Cyclic GMP is formed from GTP by guanylyl cyclases and degraded to GMP by a phosphodiesterase. The levels of phosphorylation of protein kinase A substrates (such as phosphorylase kinase and CREB) are thus determined by a balance between the intracellular activities of protein kinase A and protein phosphatases.Ĭyclic GMP (cGMP) is also an important second messenger in animal cells, although its roles are not as clearly understood as those of cAMP. For example, the serine residues of proteins that are phosphorylated by protein kinase A are usually dephosphorylated by the action of a phosphatase called protein phosphatase 1 ( Figure 13.22). These protein phosphatases serve to terminate the responses initiated by receptor activation of protein kinases. A number of others are cytosolic enzymes that remove phosphate groups from either phosphorylated tyrosine or serine/threonine residues in their substrate proteins. Some protein phosphatases are transmembrane receptors, as discussed in the preceding section. To the contrary, protein phosphorylation is rapidly reversed by the action of protein phosphatases. It is important to recognize that protein kinases, such as protein kinase A, do not function in isolation within the cell.
#Signal transduction free#
The free catalytic subunit of protein kinase A translocates to the nucleus and phosphorylates the transcription factor CREB (CRE-binding protein), leading to expression of cAMP-inducible genes. Such regulation of gene expression by cAMP plays important roles in controlling the proliferation, survival, and differentiation of a wide variety of animal cells.Ĭyclic AMP-inducible gene expression. Within the nucleus, protein kinase A phosphorylates a transcription factor called CREB (for CRE- binding protein), leading to the activation of cAMP-inducible genes. In this case, the signal is carried from the cytoplasm to the nucleus by the catalytic subunit of protein kinase A, which is able to enter the nucleus following its release from the regulatory subunit. In many animal cells, increases in cAMP activate the transcription of specific target genes that contain a regulatory sequence called the cAMP response element, or CRE ( Figure 13.21). Hormone binding to a small number of receptors thus leads to activation of a much larger number of intracellular target enzymes. Signal amplification continues as each molecule of protein kinase A phosphorylates many molecules of phosphorylase kinase, which in turn phosphorylate many molecules of glycogen phosphorylase. Each molecule of G s then stimulates the enzymatic activity of adenylyl cyclase, which can catalyze the synthesis of many molecules of cAMP.
However, each receptor may activate up to a hundred molecules of G s. Each molecule of epinephrine activates only a single receptor. The chain of reactions leading from the epinephrine receptor to glycogen phosphorylase provides a good illustration of signal amplification during intracellular signal transduction. Glycogen synthase (which catalyzes glycogen synthesis) is inhibited by this phosphorylation, whereas phosphorylase (more.) Protein kinase A phosphorylates both glycogen synthase and phosphorylase kinase. Regulation of glycogen metabolism by protein kinase A.


 0 kommentar(er)
0 kommentar(er)
